You can also add to your health journal encourading news about advances in the breast cancer management. Here are some of them:
FDA approved AstraZeneca's breakthrough breast cancer therapy Enhertu.
AstraZeneca's Enhertu and Genentech's Pertuzumab combination therapy received (in 2025) Breakthrough Technology Designation from the FDA for the treatment of adult patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant treatment who are at high risk of recurrence. Enhertu is an antibody-drug conjugate that combines a gene-targeted therapy and a chemotherapy agent in a single molecule. This is the only treatment approved in over a decade that has demonstrated a statistically significant improvement in progression-free survival compared to the current standard of care for patients with HER2-positive metastatic breast cancer. With a median progression-free survival of over three years, compared to approximately two years for THP, the new combination is expected to become the new standard of first-line treatment in this field. Approximately one in five breast cancer cases is HER2-positive, which is associated with an aggressive disease course and poor prognosis.
Olaparib and chemotherapy resulted in 100% survival rate for aggressive breast cancer.
Olaparib, which is owned by the British company AstraZeneca, has been used to treat cancer since 2014. However, a new protocol of its use in combination with chemotherapy for the treatment of breast cancer (before surgery) has achieved significantly better results. The study was conducted by a team from the University of Cambridge. It involved patients with early-stage breast cancer with inherited mutations of the BRCA1 and BRCA2 genes. In result, out of 39 study participants, only one had a relapse three years after surgery. Moreover, all 100% showed a three-year survival rate. For comparison, in the control group (chemotherapy + tumor removal surgery), the survival rate was only 88%. The new protocol for drug administration is more effective and also more cost-effective due to the short treatment duration. If phase II trials involving a larger patient population demonstrate similar results, clinical guidelines will be adjusted.
AstraZeneca succeeded in treating breast cancer where Roche and Sanofi failed
Pharmaceutical company AstraZeneca scored a double victory over its competitors Roche and Sanofi. Its two promising drugs for the treatment of breast cancer in 2022 achieved the main goals of phase 3 and 2 clinical trials, while similar drugs from Roche and Sanofi have failed the same trials. The first drug, Capivasertib, an inhibitor of the AKT protein, is used to treat HR-positive, HER2-negative breast cancer and is in Phase 3. The second, the oral drug Camizestrant, a selective estrogen receptor disruptor (SERD), is used for patients with ER-positive local or metastatic breast cancer.
Novartis Kisqali: Hope for Patients with Metastatic Breast Cancer
In 2001, the Nobel Prize in Medicine was awarded for the discovery of a mechanism for suppressing tumor growth—cyclin-dependent kinase (CDK) inhibitors—compounds that disrupt the cell cycle and allow cells to divide uncontrollably. This became the starting point for the design and development of a new class of drugs for the treatment of breast cancer - CDK 46 inhibitors, which block tumor growth. Novartis used this technology to create Kisqali, the results of a phase III clinical trial for which were published in 2021. The drug was used in combination with hormone therapy and significantly increased life expectancy and quality of life. The company says that a disease that was recently fatal and fleeting now has the potential to become chronic - one that can be controlled for years.
New breast cancer treatment cuts procedure time from 2.5 hours to 5 minutes
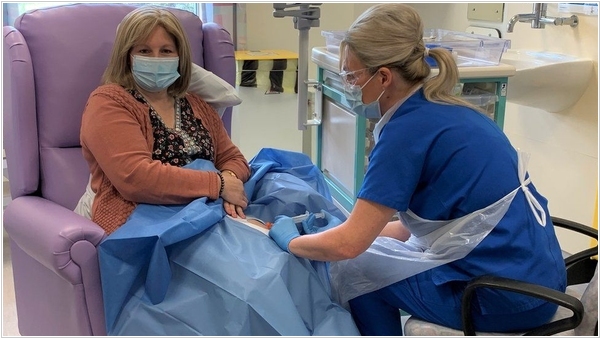
Roche's new cancer therapy, Phesgo in 2021 was approved in the US and Europe for the treatment of HER2-positive breast cancer. It's a combination of pertuzumab, trastuzumab and antibodies used in combination with chemotherapy for breast cancer. While these drugs were previously administered intravenously over 2.5 hours, Phesgo is administered subcutaneously in 5 minutes and is gentler on the body. According to Delyth Morgan, director of the UK-based Breast Cancer Now foundation, Phesgo's regulatory approval is "fantastic news" for the thousands of women who will benefit from a faster, more gentle treatment.
Hope for HER2-positive breast cancer treatment appeared
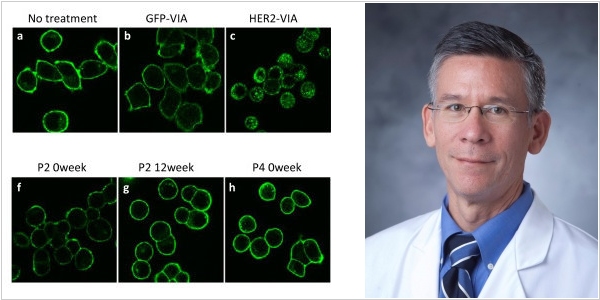
Immunotherapeutic drugs Tecentriq (Roche) and Keytruda (Merck) are effective against triple-negative breast cancer, but they are ineffective against HER2-positive breast cancer. This is because these tumors contain fewer antigenic elements that the immune system can recognize. Scientists at Duke University, led by Kim Layery, in 2020 discovered that the HER2D16 gene suppresses its expression and created a vaccine that, in turn, suppresses this gene. As a result, the combination of their vaccine and Keytruda demonstrated surprisingly high efficacy in mouse trials and paved the way for the immediate start of Phase 2 human clinical trials.
